Which One of the Following Is the Strongest Acid
A CH 3 COOH B CH 2 ClCOOH C CHCl 2 COOH D CCl 3 COOH Easy Solution Verified by Toppr Correct option is D Strong acid means weak conjugate base. Which one of the following is the strongest acid.
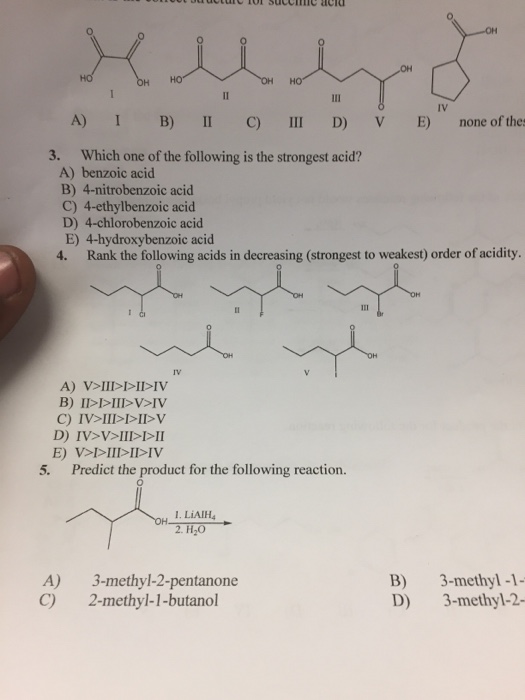
Solved Which One Of The Following Is The Strongest Acid Chegg Com
A ClCH2COOH B BrCH2COOH C FCH2COOH D ICH2COOH Option C Advertisement Advertisement New questions in Chemistry.
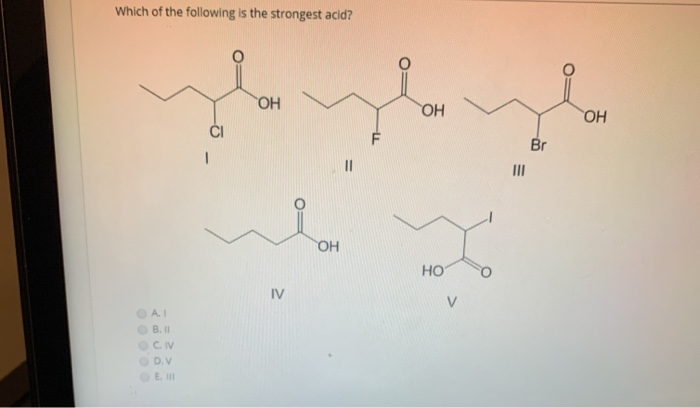
. C FCH2COOH For the most part strong acids have ions in solution so bonds holding H and A together. A ClO 3 OH B ClO 2 OH C SOOH 2 D SO 2 OH 2 Hard Solution Verified by Toppr Correct option is A Perchloric acid HClO 4 or ClO 3 OH is the strongest acid. This means that in a solution all of their molecules break up.
Of the following which is the strongest acid. Hence you get a stronger acid. FCH2COOH is the strongest acid out of all.
ACH3CH2CH2COOH bCH3CH2CHClCOOH cCH3CHClCH2COOH dClCH2CH2CH2COOH B Which of the following is the strongest base. When kept in a dry form this compound is a very potent oxidizer. It can be noted that perchloric acid is a stronger acid than sulphuric acid and nitric acid.
Consider two solutions of the weak acid HCN one with concentration 010 M and one with concentration 0010 M. HF Ka 65 x 10-4 HF Ka 65 x 10-4 Which of the following does not represent a conjugate acid-base pair. Learn vocabulary terms and more with flashcards games and other study tools.
-I and M effect in CN is more when compared to Cl I and Br. HC2H3O2 aq -- H C2H3O2- Molarity equation. This is the general order in which I want you to think of in terms of looking at the acidity of a compound.
This makes the proton less strongly attracted to any one of the oxygen atoms in the conjugate base. Strong acids are acids that completely dissociate into their ions in water. R stands for Resonance.
Of the following which is the strongest acid. D Order lies with I and M effect. Which of the following compounds is the strongest acid.
Question Which of the following is the strongest acid. Select the statements that correctly describe these solutions. Which is the stronger acid of each of the following pairs of acids.
What is the stronger acid or base. The acid strength of all of the above is the above is the same An aqueous solution at 250 degree c contains H 0099 M. The acid strength of all of the above is the above is the same An aqueous solution at 250 degree c contains H 0099 M.
CCl 3 COO. Higher the oxidation number of central atoms more acidic is the oxyacid. The conjugate base ClO 4 has maximum delocalization due to which it is weakest base.
The benzoic acid is a meta directing group and chlorine as substituents at C-2 C-3 C-4 C-5 and C-6 make the. Which of these acids is a strong acid. Since chlorine is an electron withdrawing group having negative inductive effect the acidity of the compound increases when attached to a more withdrawing group.
A I B II C III D IVE V. CH3COOH HF H3PO4 H2SO3 HI HI Select the strongest acid from the following list HBrO HBrO2 HClO2 HClO3 HIO HClO3. These are related to the letters A R I and O.
A HF b HI c mathrmHBr d mathrmHCl Answer. Which one of the following is the strongest acid. After losing H the negative ion on the C will be delocalized to CN making it more stable and acidic.
Choose your ans your choice using the correct descriptors for each of the 5 drop dow the following statement true. A ClCH 2 COOH b BrCH 2 COOH c FCH 2 COOH d ICH 2 COOH Answer. 23-dimethylheptanoic acid 33-dichlorohexanoic acid 4-bromo-3-iodohexanoic acid 4-bromo-3-chloroheptanoic acid 4-bromo-2-fluorohexanoic acid The strongest acid is Select Select This problem has been solved.
The strong acids are hydrochloric acid nitric acid sulfuric acid hydrobromic acid hydroiodic acid perchloric acid and chloric acid. Which of the following is the strongest acid. FCH2COOH ClCH2COOH BrCH2COOH ICH2COOH.
Weak acids on the other hand will. A stands for Atom. Start studying 7 Strong Acids and 8 Strong Bases.
Chlorine has highest oxidation number 7. Then which is the strongest acid in the following. HClO Ka 30 x 10-8 CHNO2 Ka 45 x 10-4 D.
Perchloric acid has many stringent regulations associated with its handling due to its extremely powerful oxidizing properties. A is more important generally than R R is more important generally than I and O is normally the last on the list. JEE Questions Which Of The Following Is Strongest Acid Which of the following is strongest acid a CHCl 3 b CHI 3 c CHBr 3 d CH CN 3 Answer.
Strong acids yield at least one hydrogen cation H per molecule. The substance NH3 is considered a weak base The substance CH3CH22NH is considered a weak base The substance HClO4 is considered a strong acid Which of the following is the strongest acid. The only weak acid formed by the reaction between hydrogen and a halogen is hydrofluoric acid HF.
The compound E is the strongest acid. HCN Ka 63 x 10-10 B. While the H 0 of pure HF is 15 addition of just 1 mol of SbF 5 lowers it to around 20.
Which one of the following is the strongest weak acid. Since the electron withdrawing inductive effect -I effect of the halogens decreases in the order of FCl Br I therefore the acidic strength of the α- halo acids decreases in the same order. Calculate the molarity of a solution containing 180 moles.
Strength of Conjugate base order. Fluoroantimonic acid is the strongest superacid based on the measured value of its Hammett acidity function H 0 which has been determined for different ratios of HFSbF 5. Question Which one of the following is the strongest acid.
Which of the following is the strongest acid. What are the strong acids. The strength of an acid simply refers to its ability to release hydrogen ions into a solution.
ANaOH bNaCO3 cH2O dCH3OH A Which of the following is the strongest base. HCl HBr HI HNO3 HClO3 HClO4 H2SO4 What are the strong bases. LiOH NaOH KOH RbOH CsOH Ca OH2 Sr OH2 Ba OH2 Strong electrolytes dissociate ___ into ions completely Weak electrolytes produce ions but predominantly as molecules that are ____ not ionized Ex.
Aiodide anion I bfluoride anion F cbromide anion Br dchloride anion Cl A.

Solved Which One Of The Following Is The Strongest Acid Chegg Com
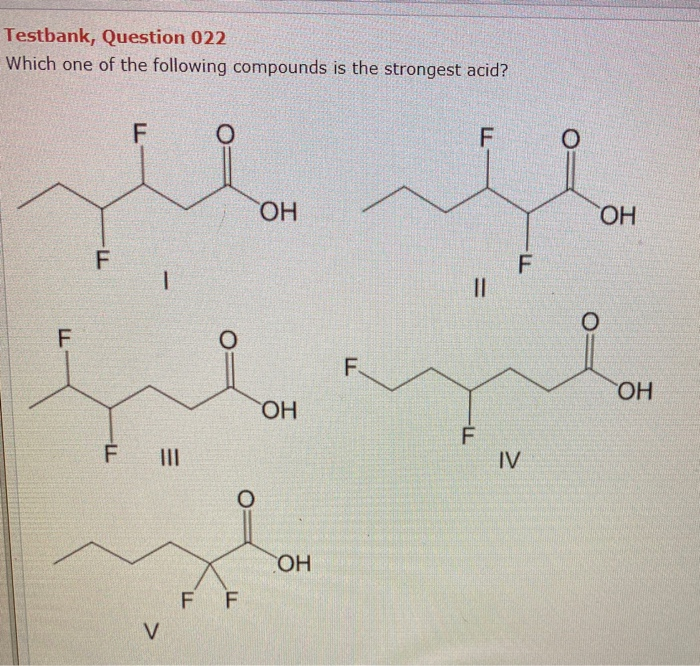
Solved Testbank Question 022 Which One Of The Following Chegg Com

Solved Which Of The Following Is The Strongest Acid On On Chegg Com

Comments
Post a Comment